Hiring: Specialists for wastewater technology (f/m/x)
31. December 2023
Forever Chemicals – PFAS (Part 2)
19. January 2024Wastewater treatment plants - no final destination for microplastics
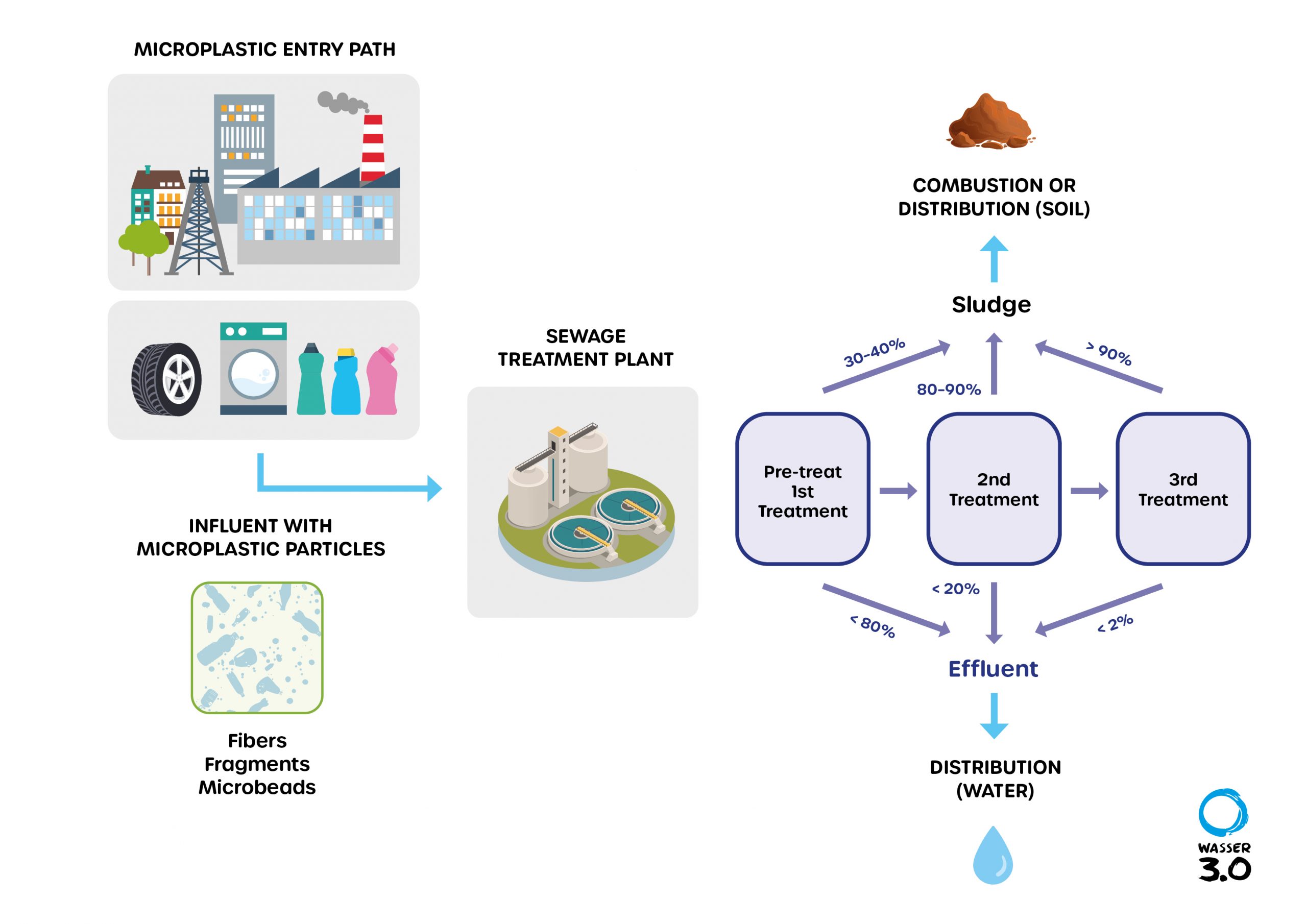
At various points in the global water cycle, sources of plastic particles into the environment can be identified. These include sewer overflows (combined sewer overflows), raw water from the separate sewer system, residual contents in treated wastewater and the recycling of sewage sludge. Our latest blog looks at the problem of microplastics in the water cycle and why sewage treatment plants are hotspots of microplastic pollution.
Wastewater treatment plants are a collection basin for microplastics
In many places, our municipal wastewater treatment plants are a collection basin for microplastics from domestic wastewater, industrial wastewater, surface runoff, rainwater, and landfills.
Although treatment plants can remove a portion of the microplastics – functioning as a partial barrier – they are still a significant source of the further distribution of microplastics into the water cycle.
The reason for this, is that they discharge large quantities of more or less purified wastewater into adjacent streams, rivers or directly into the sea every day. But many pollutants, including micropollutants and microplastics, are not removed completely or monitored comprehensively.
The problem increases when already overloaded wastewater treatment plants are confronted with heavy rainfall events and the wastewater is discharged untreated. Estimates from studies show that each sewage treatment plant transports between 93 million and 8.2 billion plastic particles into rivers and oceans every year. The water pollution contains 86 to 714 per cubic meter of microplastic particles, and fibers in the range of 98 to 1479 per cubic meter.
Furthermore, initial scientific data confirms that not only municipal wastewater treatment plants are a source of microplastics, but also industrial wastewater treatment plants. Microplastics can be released into the environment from the polymer producing and processing (plastics) industry. However, there is still a considerable knowledge gap about the role of industrial wastewater treatment plants in relation to environmental pollution with microplastics and microfibers.
What is the behavior of microplastics?
Microplastics (=synthetically produced, organic-chemical macromolecules) are characterized by the fact that they undergo no or only a very slow chemical reaction/degradation. They are also polymeric compounds to which many additives (e.g. flame retardants, UV adsorbers, plasticizers, etc.) are often added to improve their properties.
Due to the fundamentally different physicochemical properties compared to dissolved organic-chemical or inorganic-chemical stressors, the elimination of microplastics requires new techniques, as the techniques of the current fourth purification stage and even more so the state of the art with three or fewer purification stages are not sufficient.
The difficulties of microplastic removal
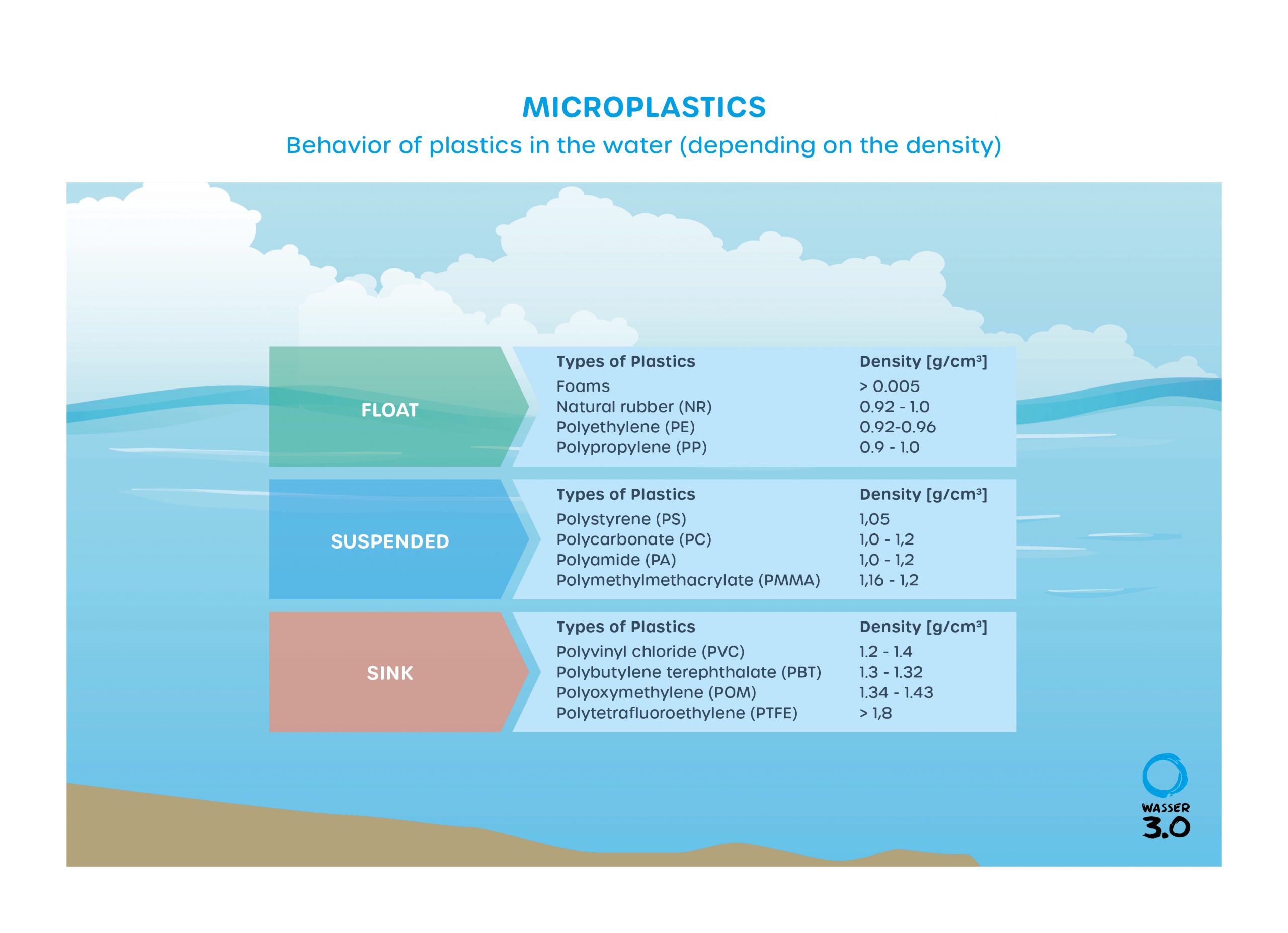
When a polymer enters the aquatic environment (fresh or salty water), this usually leads to a swelling of the polymer, so that the shape of an object can change, and its strength and dielectric properties can be reduced. In addition, the density changes, which leads to sinking or floating of the polymers or even a slow suspension in the water column.
These effects are strongly influenced by external factors (e.g. temperature, water quality) within environmental processes.
Water absorption can also lead to the breaking of chemical bonds in the polymer molecule, although this process generally only occurs at high temperatures and with polymers obtained by polycondensation at a considerable rate and is thus hardly observed in the environment.
Microplastic removal technologies
Currently, only very cost-intensive, and inefficient processes such as micro-, nano- and ultrafiltration or reverse osmosis processes are used to remove microplastics from wastewater. Up to now, these have only been able to ensure a small and limited degree of particle separation.
Microfiltration and ultrafiltration, for example, cannot retain nutrients and trace substances such as sulphates, chlorides, nitrates, pesticides, or humic substances.
Nanofiltration and reverse osmosis can remove these, but in a "dead-end filtration" application, they are quickly covered with a layer of additional dirt particles. Continuous cleaning and rinsing intervals are mandatory to provide the needed cleaning results. This leads to high operational costs . The "cross-flow filtration" method is an alternative, but carries a higher risk of retained trace substances being flushed out than the "dead-end method". Additional factors are the large structural and spatial capacities required, and high investment and maintenance costs.
Only by combining membrane filtration with other process steps can a sufficient flow and elimination performance be achieved. For example, the Oldenburg wastewater treatment plant uses a cloth filter filtration process. There, it is possible to reduce 97% of the total load (from 1131 to 29 microplastic particles and fibers (unit MP / m³) and to ensure the retention of plastic particles up to a certain size (depending on the pore diameter). A disadvantage of this process is that the filters can quickly become clogged, making backwashing necessary, which interrupts the filtering and cleaning processes. Mechanical stress on the cloth filters generates additional microplastic particles and fibers, which represent a so-called secondary source.
Wasser 3.0 PE-X® provides technological levers and solutions for water without microplastics
In a long-term trial at the municipal wastewater treatment plant in Landau / Pfalz, we were able to detect and technologically remove microplastics for the first time over the course of a year. The project delivered remarkable results. sing our standardized detection and sampling method, microplastic levels were found to range from 6 to 62 microplastics/L, and with our Wasser 3.0 PE-X ® technology, we could remove an additional 61% of microplastics, with ongoing optimization to increase removal rates to >80%. Industrial wastewater trials have achieved microplastic removal rates of 99.6%. The technology has low energy and maintenance requirements, and the removed agglomerates can be reused as e.g. construction infill material for a circular and sustainable process. These projects show once again how important it is to drive the issue of microplastics in the environment forward in a data-driven manner and not to rely on estimates or outdated, non-comparable data sets, and to target microplastic removal upstream at the source.
At Wasser 3.0, we base our solutions on standardized detection. This allows us to identify the hotspots of microplastic pollution and apply our circular economy microplastic removal in a targeted and precise manner.
Does this sound exciting to you and like a new way to curb the microplastics crisis? Then take the opportunity to work with us today to develop your tailor-made solution for water without microplastics.