From everyday helper to environmental problem: Polymers, Synthetics, Plastics & Co
Synthetics - colloquially also known as plastics - are used worldwide in a wide range of consumer goods such as clothing, household items and toys. In industrial and commercial production, they are elementary components. In 2018, around 360 million tons of plastic were produced worldwide. This means that the pure production volume of plastics has increased sevenfold worldwide since the 1970s, and the trend continues to rise massively.
The diverse and excessive use of plastics causes high amounts of plastic waste. Although a large part is landfilled or recovered in waste incineration plants, and a small part is also recycled, enormous quantities are released into the environment through improper and inadequate disposal, leakage or other losses.
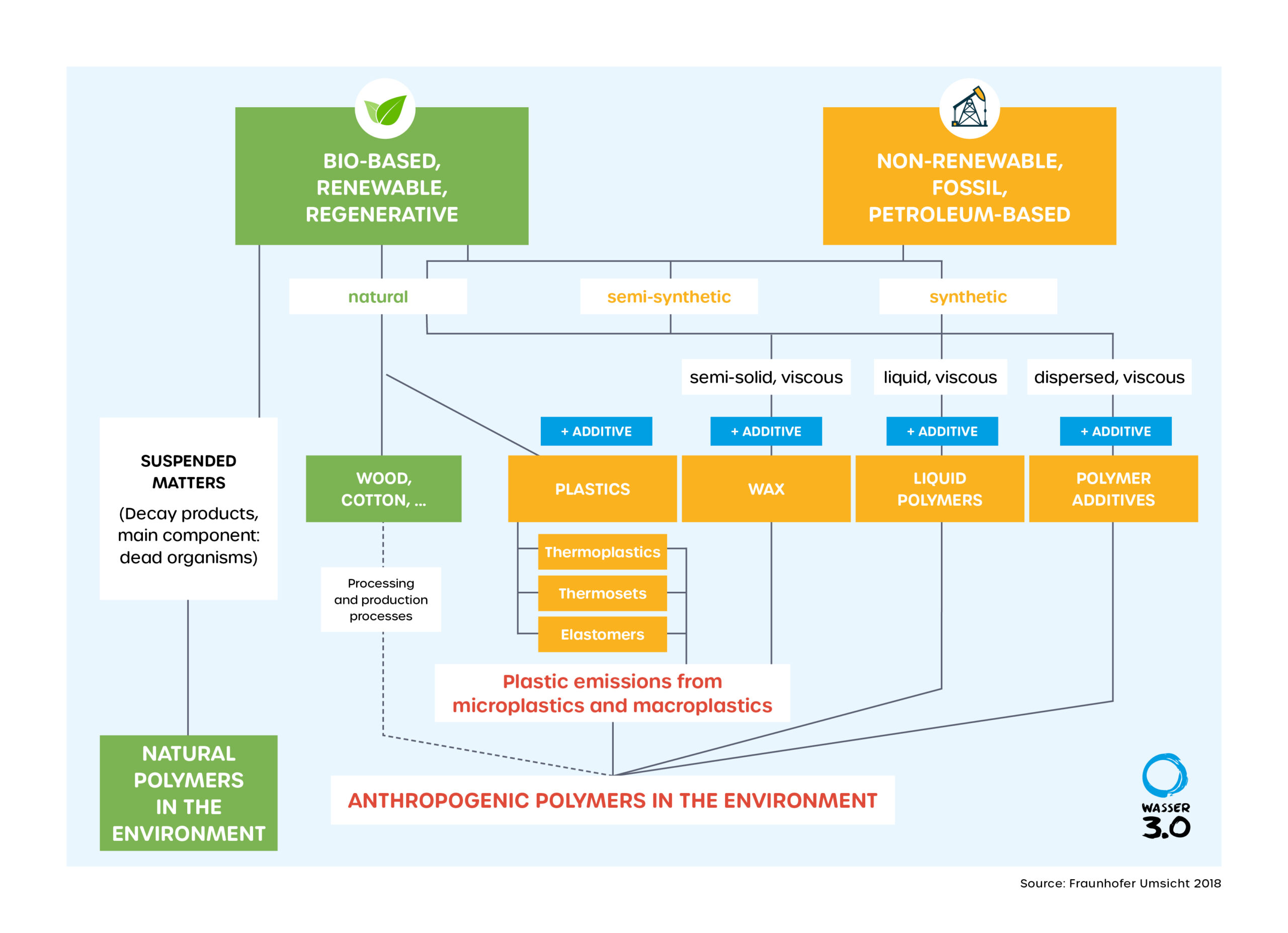
What are polymers?
Polymers are long-chain molecules composed of smaller repeating units called monomers. Some polymers contain only one type of monomer building blocks; others, known as copolymers, may contain two or more different types of monomers.
The arrangement of polymer chains over multiple length scales and a detailed understanding of the properties of plastics, including their degradation and different structures, is a key challenge in polymer science. We have compiled some essential information here.
How are polymers produced?
YouTube content is currently blocked to protect your data. By unblocking the video, you accept YouTube's privacy policy.
More informationHow many different polymers are there?
There are around 200 different types of polymers, including well-known representatives such as polyethylene, polypropylene and polystyrene. These in turn have the most diverse subcategories and are processed into millions of products. Plastic-based products obtain their specific functionalities and application-related properties by mixing further chemicals ("additives") with the polymers. Some of these include highly toxic substances such as plasticizers, water-repellent perfluorinated and polyfluorinated compounds, and bisphenol A.
What are synthetics?
Synthetic materials are made up of synthetic polymers. They make our modern life possible. Together with various additives (such as stabilizers, flame retardants and plasticizers) that influence the physical properties of the material. They are light and resilient, can be processed into fibers and films, but also into large components, foamed and shaped into any conceivable form.
YouTube content is currently blocked to protect your data. By unblocking the video, you accept YouTube's privacy policy.
More informationWhat is plastic?
Plastic is the colloquial term for synthetics. All kinds of synthetic materials are subsumed under the term plastic. Plastics are mainly made from petroleum (fossil raw materials) with many additives, some of which are toxic. The most commonly used plastic is polyethylene.
We often speak of plastic when we speak of synthetic waste, while in the production and processing of synthetic polymers, the product is usually described as a synthetic product.
What are (water-)soluble or liquid polymers?
YouTube content is currently blocked to protect your data. By unblocking the video, you accept YouTube's privacy policy.
More informationWhere are soluble polymers used?
Today, synthetic water-soluble polymers are an indispensable component of many everyday products
They are added to many cosmetics, hair sprays, creams and lotions (summarised as personal care and cosmetic products; PCCPs). In the medical field, they are used as film material for retard capsules, as binders for medical materials and as active ingredient components.
In paper production, polymers are added as additives to ensure that the white pigments are finely and evenly distributed on the cellulose fibres. Another application of soluble polymers is their use to improve the weaveability of textile fibres during the sizing process.
Polymers are added to detergents to prevent the build-up of limescale on textiles and washing machine heating rods. The paint and coatings industry uses water-soluble polymers as pigment dispersants and rheological modifiers.
Water-soluble polymers are also used as flocculants or flocculation aids in water treatment and waste water treatment. In agriculture, they help to increase the effectiveness of pesticides and fertilisers, thereby reducing the consumption of resources.
Another application for water-soluble polymers is in the construction industry, e.g. as an additive for concrete in order to achieve the highest possible degree of compaction.
How do soluble polymers work?
Polyvinyl alcohol (PVAL or PVOH) is one of the most widely used water-soluble polymers.
It is used due to its layer-forming, emulsifying and adhesive properties. It also has high tensile strength and flexibility.
Due to its hydrophilic properties, PVAL can absorb water, which acts as a plasticiser. PVAL thus loses tensile strength with increasing water content and gains elasticity in the process. Polyacrylamide (PAM) is another frequently used water-soluble polymer.
In low concentrations it can increase the viscosity of aqueous solutions, in high concentrations it forms solid gels.
How do soluble polymers get into the environment?
Just like insoluble polymers - microplastics - water-soluble polymers are also introduced directly into the environment: via plastic waste (e.g. incompletely emptied shampoo bottles), exfoliants and additives in cosmetics and household cleaners or detergents that are introduced into the water cycle during swimming or washing, for example, and transported within it.
In the sewage treatment plant, water-soluble polymers are partially extracted from the wastewater, while the remaining polymers are released into the environment with the treated wastewater.
Indirect entry paths can be caused by rain events that wash out the polymer particles from coatings and other products. In addition, the soluble polymers removed from the wastewater in the sewage treatment plant end up in the sewage sludge and on agricultural fields if it is used there as fertiliser. As with microplastics, the sewage treatment plant can be seen as an important pathway for soluble polymers to enter the water cycle and the environment.
In contrast to microplastics, there is no data on the distribution of soluble polymers in the various environmental compartments (water - air - soil) and their fate is largely unknown.
As they are dissolved in water, easily attach to other substances in water and are usually not biodegradable, it can be assumed that soluble polymers also represent a global environmental problem with high health risks.
What is the environmental relevance of water-soluble polymers?
As the water-solubility of organic chemical compounds such as polymers enables their extensive transport in aquatic ecosystems, water-soluble polymers are increasingly being found in water and soil samples.
Just like microplastics, they act as anthropogenic stressors, degrade very slowly in the environment and affect organisms and ecosystems worldwide.
What categories can soluble polymers be divided into?
Synthetic water-soluble polymers can be categorised into four groups. These include
- polyelectrolytes,
- amphoterics,
- non-ionic homopolymers and
- hydrophobic-associating polymers.
Polyelectrolytes are polymers that have charged groups. They are categorised as polycations (positively charged), polyanions (negatively charged) or amphoteres (zwitterions, positively and negatively charged). Polyelectrolytes include proteins, but also numerous synthetically produced additives for thickening cosmetics. These include polyacrylate (cabomer), polyquaternium 6 and polyvinylpyrrolidone (PVP).
The group of water-soluble non-ionic homopolymers includes, for example, polyethylene oxides, poly- N-vinylpyrrolidones, polyvinyl alcohols and polyacrylamides.
What are biopolymers?
The term "natural polymers" could lead to misunderstandings. Are there also unnatural polymers? The reason why this group of substances is called "natural" is because they originate from nature, i.e., they are polymers of biological origin; they can also be more accurately described by the term "biopolymers".
What is bioplastic?
Bioplastics are both plastics that are biodegradable and plastics made from renewable raw materials (biopolymers). These do not necessarily have to be biodegradable - for example, natural fiber-resin mixtures for coffee cups made from bioplastics. Bioplastics is not a protected term!
When does plastic become microplastic?
As soon as plastic particles smaller than 5 mm enter the environment (air - soil - water), no distinction is made between 200 individual plastic types or products. The multitude of different polymers, including associated additives (e.g. plasticizers, PFAS) or otherwise absorbed (toxic) substances (e.g. heavy metals, pesticides, pharmaceuticals), is summarized under the term microplastics.
What does biodegradable mean?
A product is only biodegradable when microorganisms are able to convert the respective material into its elementary components such as carbon, oxygen, hydrogen and other minerals. The time factor needed for this decomposition process is irrelevant here.
Whether a material is biodegradable does not depend on the raw material or the raw material composition, but on the chemical structure of the product. As a result, plastics made from fossil raw materials are in principle also biodegradable, since they have a basic organic chemical structure. Conversely, products made from renewable raw materials can have a structure that excludes biodegradability.
If organisms are not able to decompose the materials into their components (= biodegrade), fragmentation occurs. This process is influenced by:
- pressure
- UV radiation
- salty water
- temperature.