Micropollutant special: Phosphonates - released into the environment via detergents and cleaning agents, pesticides and antiscalants
For several decades, phosphonate groups have been included as functional groups in a growing number of chemicals. They were originally developed for thermal wastewater treatment but are now also used in membrane processes.
Synthetic phosphonate compounds have become ubiquitous constituents of natural waters and wastewaters due to their diverse applications in industry and agriculture.
Properties of organic-phosphorous compounds, e.g. phosphonates
Phosphonates are organophosphorus complexing agents with direct phosphorus-carbon bonding. Phosphonates exhibit various properties, e.g. excellent sequestering ability, i.e. complex formation with metal ions under stoichiometric conditions, prevention of precipitation of poorly soluble alkaline earth compounds ("hardness"), e.g. calcium carbonate, calcium sulfate or barium sulfate under stoichiometric conditions (threshold effect).
In addition, they have very good dispersing properties, i.e. stabilization of dispersed solids in suspensions and slurries, prevention of undesirable flocculation, excellent hydrolytic stability even at high temperatures and over a wide pH range.
Phosphonates are used in engineering as corrosion inhibitors, for process water treatment and as peroxide stabilizers. Peroxide stabilizers are contained in small quantities in detergents containing bleach.
Phosphonate-based chelates are used to prevent the occurrence of unwanted precipitation and crystal growth. This effect already occurs at concentrations below the amount required to convert all metals into complexes.
Distinguishing features in phosphonates
Phosphonates are a group of organic compounds containing one or more phosphonic acid groups. Basically, a distinction can be made between mono- and polyphosphonates.
At strongly acidic pH values, the phosphonates are completely protonated. With increasing pH, the phosphonates are gradually deprotonated according to their dissociation constants. While the first proton is easily and completely split off, all other protons are split off more heavily and incompletely.
Monophosphonates are only partially soluble in water, while polyphosphonates are very soluble in water. The synthetically produced compounds, which are foreign to humans (so-called xenobiotic), and their metabolites can have negative effects on the aquatic environment; the delayed release of ortho-phosphate can promote eutrophication. Monophosphonates are commonly used as biocides, the best known being the herbicide N-(phosphonomethyl)glycine, also known as glyphosate, its degradation product aminomethylphosphonic acid (AMPA).
Direct and indirect risks to aquatic life depend on the dosage and the structure of the phosphonate. Acute and chronic toxicities cannot be excluded. In view of the extension of the authorization of biocides containing N-(phosphonomethyl)glycine, diffuse discharges into waters of the European Union (EU) will therefore continue to occur.
Polyphosphonates such as ethylenediaminetetramethylenephosphonic acid (EDTMP) or aminotrismethylenephosphonic acid (ATMP) are used in many industrial and household applications such as membrane processes, desalination plants, paper and oil industries and detergents.
They act mainly as complexing agents and chelating agents. The stability of the metal complexes increases with the number of phosphonate groups. They suppress crystal growth (scaling), act as precipitation inhibitors and are stable under a wide range of physicochemical conditions against thermal decomposition, hydrolysis and photolysis. Phosphonate groups, like phosphate groups, have a strong nucleophilic character, which leads to a high sorption affinity for electrophilic surfaces.
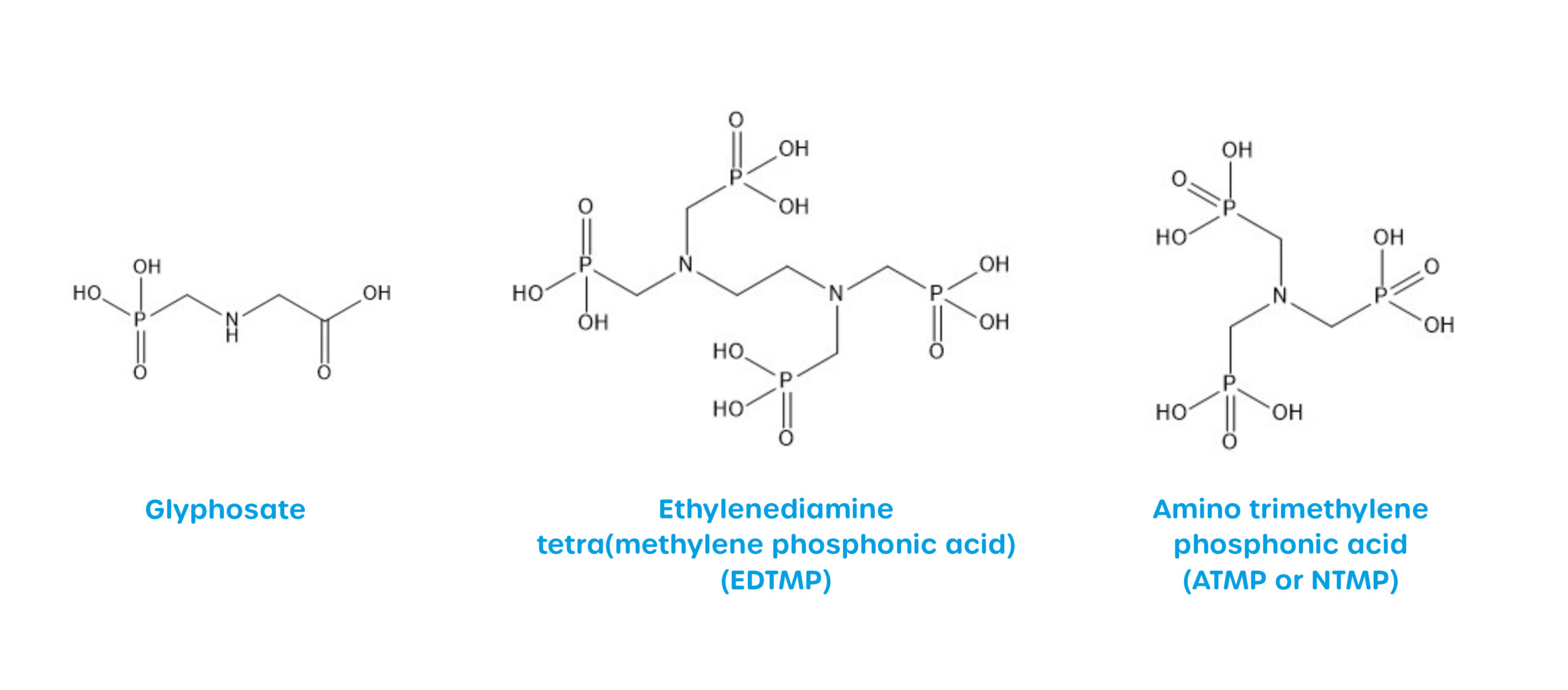
Structural formulae of selected phosphonates
What should you know about the substance class of plasticisers, flame retardants and lubricant additives (phosphoric acid triester (trialkyl, triaryl and alkyl/aryl phosphoric acid esters))?
Compounds belonging to this class of organic chemical phosphorus compounds can be both water-insoluble and water-soluble. Some compounds of this substance class have a faint aromatic odour.
Their toxicity ranges from very toxic (old classification according to the Substances Directive) to category 1 with regard to specific target organ toxicity (new GHS classification according to Regulation (EC) 1272/2008) for tri-o-cresyl phosphate, to no classification.
Some phosphoric acid esters contain halogen atoms which additionally increase the flame retardant effect (e.g. TCEP:
Tris(2-chloroethyl)phosphate; TCPP: Tris(2-chloroisopropyl)phosphate)). According to TRGS 905, TCEP is classified as carcinogenic and toxic to fertility category 2.
Further information on substance properties can be found in the
German Social Accident Insurance's hazardous substance information system, the GESTIS substance database.
Phosphonates in the environment
Although some phosphonates also occur in nature, their biodegradability is considered poor. Even under anaerobic (oxygen exclusion) conditions, they do not show good degradation rates. They are also considered to be moderately toxic to aquatic life.
The low level of knowledge about phosphonates is due to the fact that they cannot be detected in trace concentrations in water, or can only be detected with great difficulty. In 2021, the Federal Environment Agency conducted a study on wastewater and sediment samples, in which the analytical methods were also further developed. Method development focused on the extraction of phosphonates from solid samples, the adaptation of chromatography for the analysis of matrix-loaded samples, and the establishment of automatic enrichment for the quantification of surface water samples. However, the values do not match the values of the investigations of the DVGW-Technologiezentrum Wasser TZW (DVGW Water Technology Center) between 2015 and 2018. The question of accumulative properties in the environment as well as real removal efficiencies within treatment stages of a wastewater treatment plant can therefore not be answered unambiguously at present. Furthermore, neither an increased nor no impairment of the environment by synthetic phosphonates can be assumed or excluded.
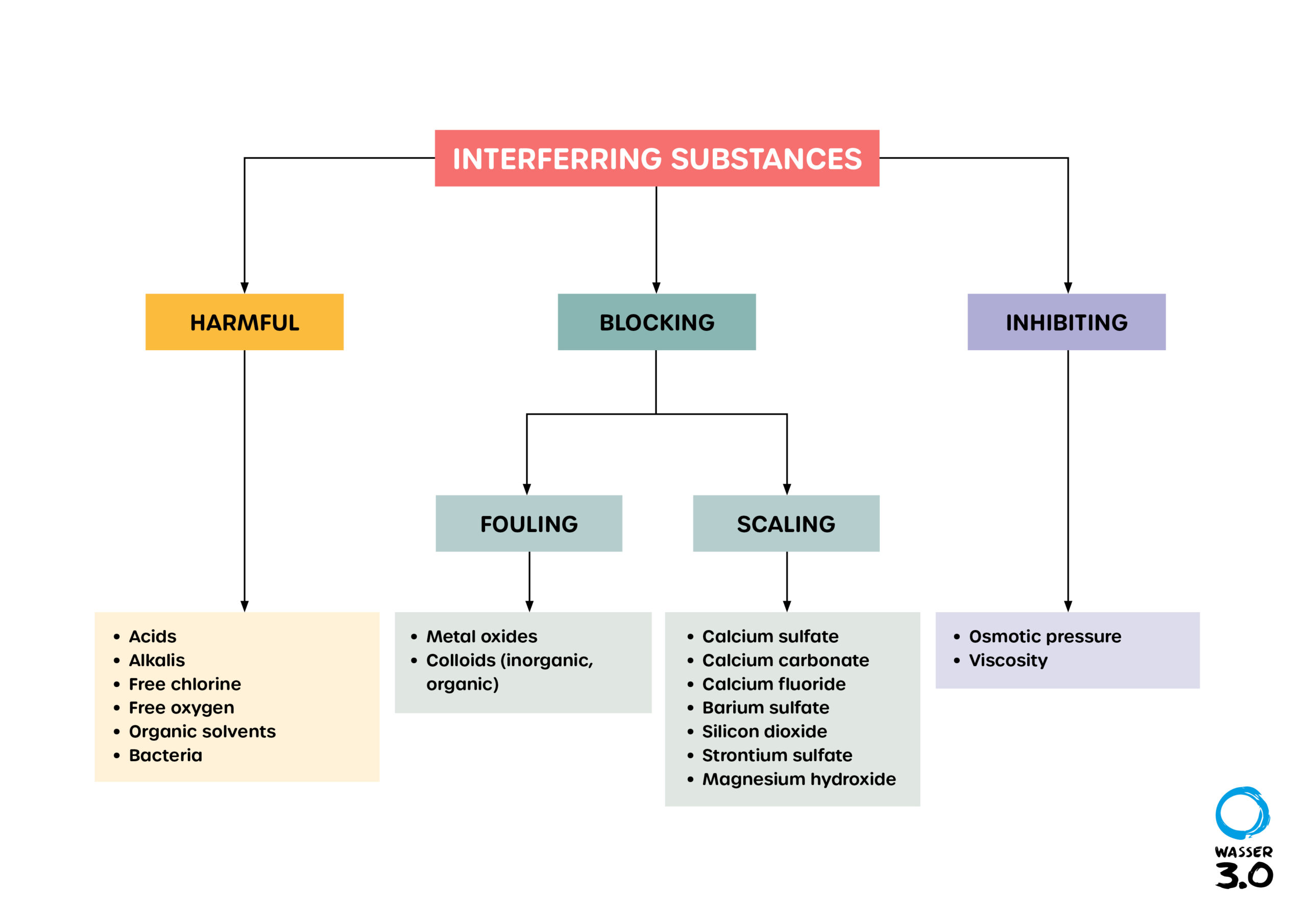
What is scaling?
Scaling is based on deposits of mineral compounds or salts that accumulate on the surface of the reverse osmosis membrane, growing there and reducing its flow rate. Membranes have a great importance in water purification. Membranes reduce the high TDS content (sum of solids dissolved in a solution) in water. Defective membranes due to deposits cause not only high costs, but also a reduction in water quality.
The concentration of these minerals in the water depends on the raw water (feed water). The mineral scalants have different influence on the scaling potential. Calcium carbonate (CaCO3), calcium sulfate (CaSO4), strontium sulfate (SrSO4) and barium sulfate (BaSO4), among others, have a major influence. Low scaling behavior is found in calcium phosphate [Ca3(PO4)2] and calcium fluoride (CaF2); silica (SiO2) is an exception. They are classified as non-harmful for most processes.
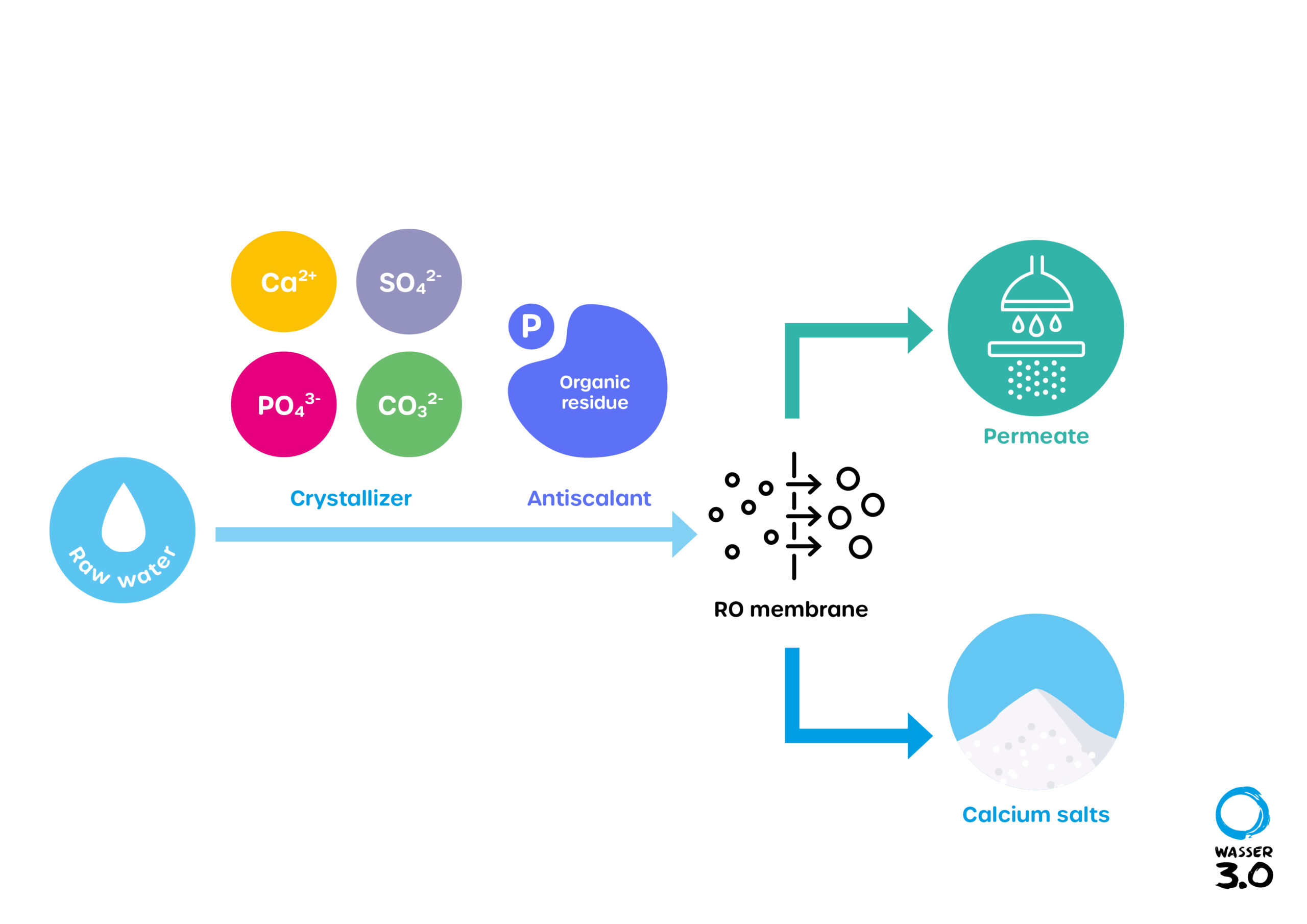
What are antiscalants?
To prevent a wide variety of deposits in pipes, heat exchangers and on membranes, so-called antiscalants (anti-fouling agents) are used. They are also referred to in simplified terms as chemical additives. Well over 100 different antiscalants have been approved for use in drinking water. Antiscalants were originally developed for thermal wastewater treatment, but are now mainly used in membrane processes.
Antiscalants do not act in stoichiometric ratios; instead, they interact in complex physicochemical processes. First, they prevent the precipitation of double-positive landed cations such as Ca2+, Mg2+ or Ba2+. The complex formation between antiscalant and cations keeps the concentrate stable, despite supersaturation of the solution. In addition, they also prevent crystal growth. This prevents the so-called nucleation of the crystals. This is either stopped or the initial crystals are modified by the crystal surface adsorbing the antiscalant and converting it into a "fluffy" form.
The mechanisms of action are very complex processes, which in turn also strongly depend on the composition of the feed. The optimum dosage of the antiscalant is therefore determined empirically in each case and must be determined on a plant-specific basis. From a process engineering point of view, care must be taken to minimize scaling, but equally care must be taken not to overdose, which can result in unintended sludge formation.
Differentiation in antiscalants
There are two types of application of antiscalants
- Solid form (balls): These balls are used in household water purifiers in the housing of the pre-sediment filter.
- Liquid form: This is used in industrial reverse osmosis (RO) systems that purify thousands of gallons of water daily. The chemical anitiscalant is added with a dosing pump at a suitable dosing rate.
Balls are not used in the industrial RO system because they would have to be changed 20-30 times a day.
How does an antiscalant work?
Antiscalant impedes the precipitation of salts on the surface of the RO membrane in three different ways:
- Threshold Inhibition: Keeps salt in supersaturated form in water.
- Crystal modification: Distorts the shape of salt crystals, resulting in soft and non-adherent scaling.
- Dispersion: Does not allow ions to form crystals that deposit on the surface of the membrane.
For the optimal function of a reverse osmosis (RO) system, raw water treatment is essential to minimize the influence of interfering substances.
There are mainly three methods to control the scaling process
What are Fouling, Biofouling und Scaling?
Disruptive processes are divided into fouling, biofouling and scaling. They primarily refer to the blocking of membranes.
- Fouling is mainly caused by colloids or metal oxides contained in the raw water, which are destabilized and precipitate on the membrane when concentrated.
- In biofouling, microorganisms are deposited on the membrane, which in turn secrete substances that embed themselves in the membrane and thus block it.
- Scaling describes the precipitation of previously dissolved salts by exceeding the solubility product and is the key process requiring the use of antiscalants. Scaling in pipes and boilers is identical to that of scale.
Antiscalants are used to prevent fouling and (bio)fouling and thus protect the susceptible membrane.