Micropollutant special: Per- and polyfluorinated compounds (PFAS) in us and our environment
Discovered as early as the late 1930s, PFAS chemistry has been an integral part of production processes and consumer goods since the 1950s. Its properties bring enormous benefits to many products that shape our everyday lives.
At the same time, they pose an enormous threat to our health and that of our ecosystems. Here we take a close look at what perfluorinated and polyfluorinated alkylsulfonates – or PFAS – are all about.
What does PFAS mean?
Per- and polyfluorinated chemicals (PFCs), which also include PFASs, are a group of synthetically produced organofluorine compounds in which the hydrogen atoms on the carbon chain have been partially or completely replaced by fluorine atoms. The strong carbon-fluorine bond (C-F: 489 kJ/mol, cf. C-H: 413 kJ/mol, C-C: 348 kJ/mol) gives the compounds water-, oil- and dirt-repellent properties with simultaneous chemical and thermal stability and resistance to UV light and weathering.
Where exactly is PFAS contained?
Due to their properties, PFAS are frequently used in surface finishing, paper coating and specialty chemicals. In electroplating, they serve as wetting agents and mist inhibitors, and in plastics production as emulsifiers. Furthermore, PFASs are components of the following everyday items, among others: textiles (Gore-Tex®), impregnating agents for surfaces of furniture, textiles, leather and carpets, dirt-repellent papers, non-stick cookware, wall paints, inks, varnishes, cleaning agents, films, photographic plates and extinguishing foams for firefighting.
Global production and emissions were first listed in a 2009 review by Paul et al. Due to their anthropogenic origin, PFAS cannot be degraded in the environment, so that they can now be detected ubiquitously in the environment.
What are PFAS chemicals actually?
The two PFAS compounds perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) are the most commonly produced, most abundant, and most studied compounds. Up to 45,250 metric tons of PFOS were produced between 1972 and 2002, and up to 7,300 metric tons of PFOA were produced between 1951 and 2004.
For many years, there have been scientific, regulatory and public concerns about potential health and environmental impacts associated with the manufacture of PFAS chemicals and the production, use and disposal of products containing PFAS. These concerns have led to efforts to reduce or replace the use of certain PFASs, especially PFOS and PFOA.
REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) has now classified PFOS as persistent (P), bioaccumulative (B) and toxic (T) . PFOS and its derivatives have therefore been listed in the Stockholm Convention for banned substances since 2010. In the EU, the use of PFOA has been banned since 2020.
How many PFAS compounds are there?
The number of PFAS and their applications have increased over the years. It is estimated that the PFAS family includes approximately 5,000-10,000 chemicals. A 2018 inventory of PFASs conducted by the OECD identified Chemical Abstracts Service (CAS) registration numbers for more than 4,700 PFAS worldwide. It is not known how many of these are actually in use.
Publicly available health and toxicity studies are limited to a small fraction of PFASs. Commercially available analytical technologies typically identify only about 20 to 30 PFAS.
Many literature references use a blanket figure of more than 3000 substances. We have also adopted this value on our PFAS fact sheet.
How do PFAS compounds enter the environment?
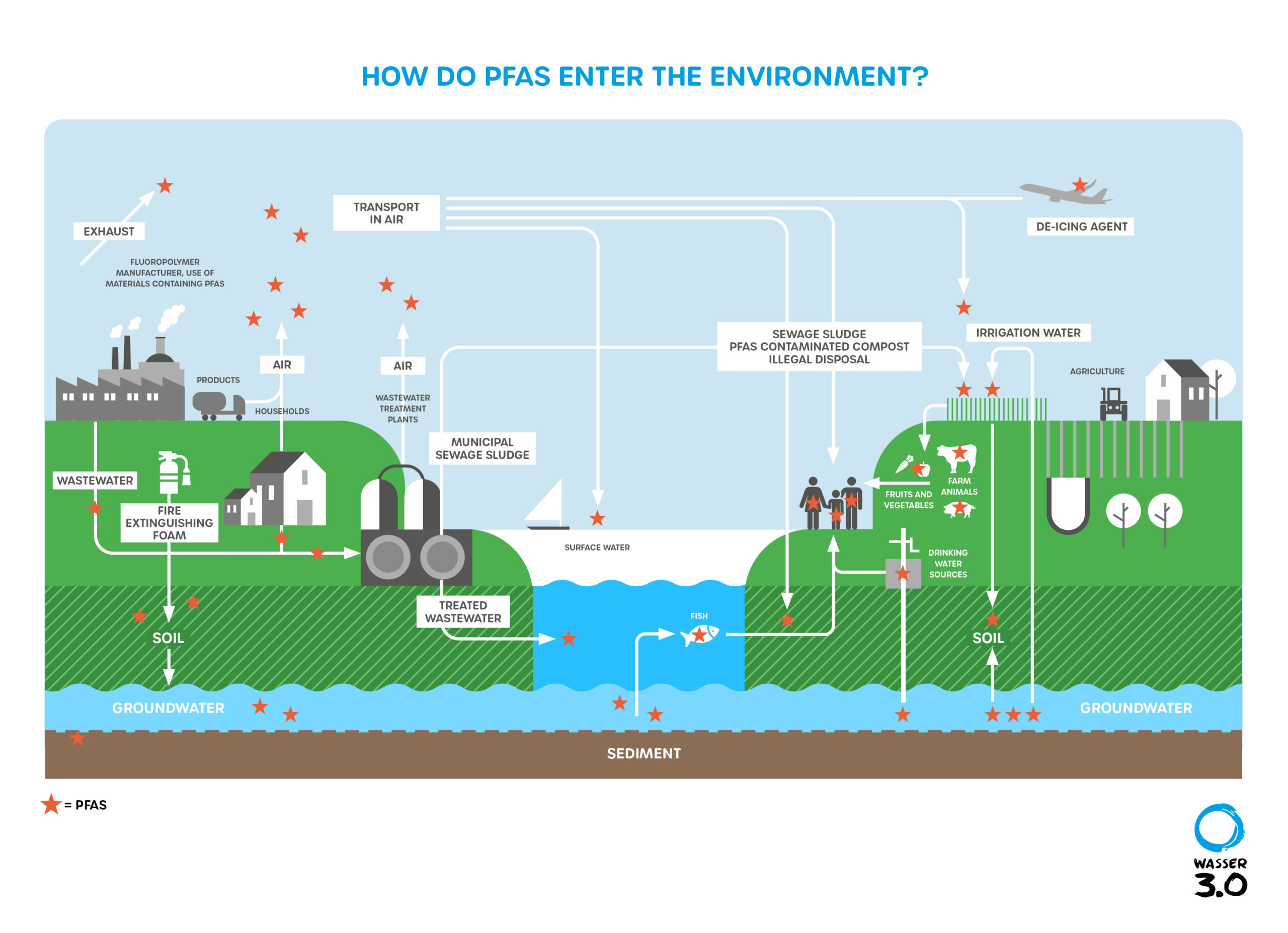
PFAS enter the environment mainly through industrial wastewater and exhaust air containing PFAS, use of PFOS-containing firefighting foam (PFOS = perfluorooctane sulfonic acid) by firefighters, and application of PFAS-contaminated sewage sludge. Indirect entry pathways represent the biotransformation in the environment of precursors such as fluorotelomer alcohols to perfluorocarboxylic acids.
The environmentally relevant point of origin during the PFAS life cycle
The raw materials are used in a variety of manufacturing processes and industrial/commercial applications to produce commercial and consumer products that contain or have been treated with PFAS. During this life cycle, different types and amounts of PFAS can be released to the environment from manufacturing waste streams, diffuse emissions, spills, disposal of PFAS-containing or -treated micropollutants, and general wear and tear of consumer products.
Sometimes the intended use of the PFAS product (e.g., firefighting foams) results in a direct release to the environment. PFAS from a variety of sources can also aggregate in wastewater treatment plant effluents and sludges, creating secondary sources of release.
The volume, concentration, and mixture of PFAS released to the environment will vary depending on the source (process, material, or product), release mechanism(s), and environmental controls applied during this life cycle. Exposure to PFAS may occur as
- direct interaction with the manufacturing process,
- professional or intensive use of materials containing PFAS,
- use of or contact with commercial and consumer products containing PFAS, or
Exposure (human or environmental) to environmental media affected by PFAS. The relative importance of these exposures also varies greatly, as can be seen in the following figure.
Notes on the graphic: The pathways shown in the figure are not fully comprehensive with respect to all sources or release mechanisms. Multiple sources may be present at a site, and the relative exposure potential and environmental impacts may vary based on several considerations.
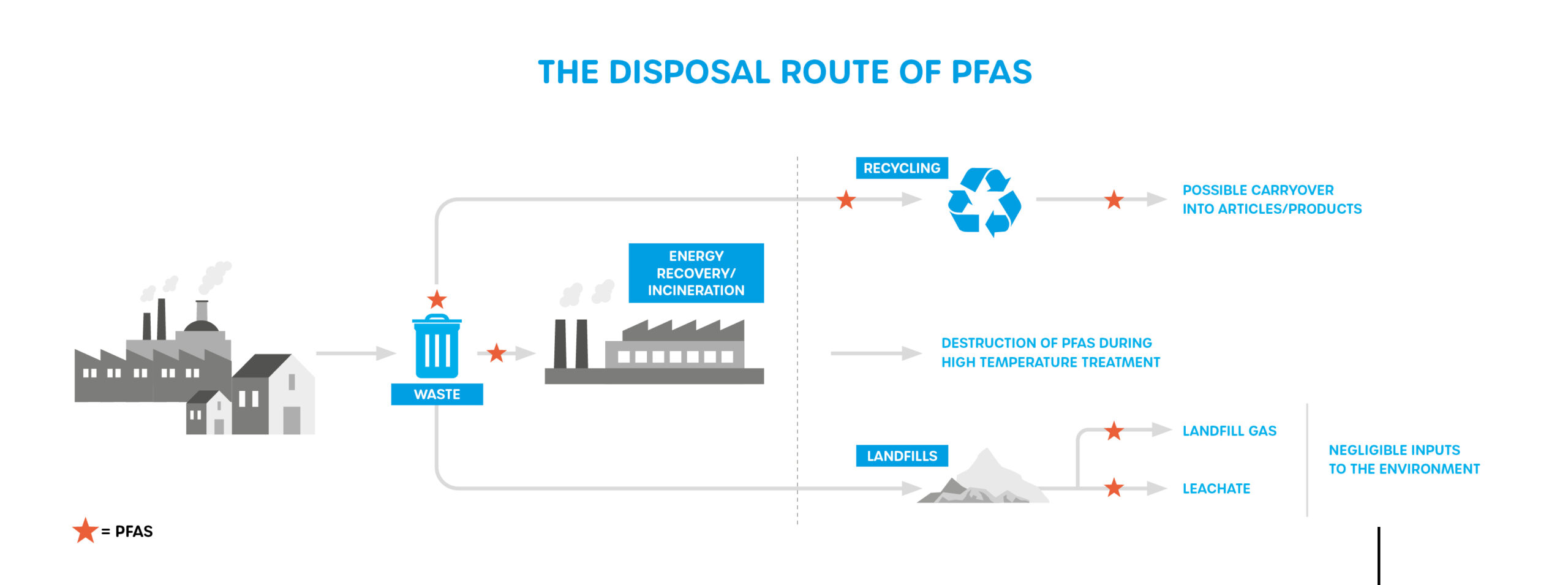
Due to the widespread use of PFAS in commercial and consumer products, other minor and diffuse releases of PFAS to the environment may occur during the use and disposal of some PFAS-containing products. Although these can result in locally significant environmental impacts, these releases typically affect smaller geographic areas and have a lower total PFAS mass than major sources, such as the manufacture of PFAS chemicals, the use of PFAS in certain industries, and the application of certain firefighting foams.
Different PFAS products and sources vary in their relative environmental significance, amounts released, distribution mechanisms, area affected, and relative concentration of media affected. For example, the application of Class B fire suppression foam may affect a moderate area relative to air distribution during fluoropolymer production, but have higher groundwater concentrations near the source area.
Notes on the graphic:
The pathways shown in the figure are not fully comprehensive with respect to all sources or release mechanisms. Multiple sources may be present at a site, and the relative exposure potential and environmental impacts may vary based on several considerations.
Due to the widespread use of PFAS in commercial and consumer products, other minor and diffuse releases of PFAS to the environment may occur during the use and disposal of some PFAS-containing products. Although these can result in locally significant environmental impacts, these releases typically affect smaller geographic areas and have a lower total PFAS mass than major sources, such as the manufacture of PFAS chemicals, the use of PFAS in certain industries, and the application of certain firefighting foams.
Different PFAS products and sources vary in their relative environmental significance, amounts released, distribution mechanisms, area affected, and relative concentration of media affected. For example, the application of Class B fire suppression foam may affect a moderate area relative to air distribution during fluoropolymer production, but have higher groundwater concentrations near the source area.
Which processes and areas are relevant when analysing the behaviour and transport routes of PFAS?
How PFAS behave in the environment when released there depends on many factors. These include the physical, chemical, and biological processes that influence the distribution of PFASs in different media, as well as the extent of migration within and between media (e.g., plume development, groundwater discharge to surface water).
The type of PFAS involved determines the relative environmental significance
Non-polymer PFAS (both per- and polyfluorinated) and some side-chain fluorinated polymer PFAS are likely to pose a greater risk if released to the environment than certain fluoropolymer sources such as the fluoropolymers PTFE, fluorinated ethylene propylene (FEP), perfluoroalkoxy polymer (FEP) PFA), and ethylene tetrafluoroethylene (ETFE).
These fluoropolymers are considered polymers of low concern because they are relatively stable, insoluble in the environment, and non-bioavailable. However, the environmental impact of manufacturing some fluoropolymers can pose a significant risk if emissions are not properly controlled at the industrial site.
Releases to the environment from disposal of fluoropolymers also cannot be ruled out, as non-polymer PFAS (such as the PFAA used as polymerization aids) may be present in significant concentrations as impurities and by-products in some fluoropolymer products.
Studies suggest that fluorinated side-chain polymers and fluorotelomer-based polymers are likely to degrade into non-polymer PFAA over time. Although it has been documented that one fluoropolymer (PTFE) had not degraded, significant levels of PFAA were detected during combustion.
Factors affecting the fate and transport of PFAS can be broadly divided into two categories:
Impact of PFAS on the environment and health
The multitude of PFAS with their diverse physical and chemical properties leads to diverse environmental impacts. Valid assessments must, therefore, do justice to an enormous complexity.
PFAS have been and continue to be widely used, but not all types and uses of PFAS result in the same environmental impact and exposure. When considering potential environmental impacts of PFAS, it is important to determine not only the particular PFAS as accurately as possible, but also where and how they are released into the environment.
For example, a stable, insoluble fluoropolymer such as polytetrafluoroethylene (PTFE, also known by the brand name Teflon) may pose little environmental or health risk when included in a product as directed. However, significant environmental releases can potentially occur: If errors occur during PTFE manufacturing (e.g., if polymeric PFAS such as PFAA are not used to make the PTFE) or if products are used improperly. Such considerations can help to focus the investigation resources on important sources.
Currently, toxicological studies are still taking place for PFAS compounds. PFAS show low acute toxicity, but long-term studies showed the promotion of the development of liver, pancreas and Leydig cell tumors. The reproductive toxic effect is undisputed: PFAS have a negative effect on fertility and the hormone system of humans.
How do we evaluate the PFAS problem?
Understanding the relevant accumulation and transport processes for PFAS is critical to determining action steps and developing preparedness plans. In the first instance, it is a matter of summarizing the answers to certain key questions.
What is known about PFAS in drinking water and groundwater?
After perfluorooctanoic acid (PFOA) was first detected in drinking water in 2002 by six water utilities near the DuPont chemical company in Parkersburg, West Virginia/USA, and also increasingly in the various production facilities of 3M in Minnesota/USA, many research groups detected PFAS in a wide variety of matrices.
The United States Environmental Protection Agency (USEPA) has compiled a comprehensive data set on the occurrence of six PFAS in public drinking water. This data set is the result of required monitoring of approximately 4,900 public water systems (all large systems serving more than 10,000 people and a subset of smaller systems) for six PFAS in treated drinking water at points of entry into the drinking water distribution system.
The study was conducted between 2013 and 2015 under the Third Unregulated Contaminant Monitoring Rule (UCMR3) and included results from treated water originating from groundwater wells (n = 22,624), surface water (n = 12,733), and mixed sources (n =) 792). A summary of the UCMR3 occurrence data, including analytical reports, can be read here.
What is known about PFAS in surface waters?
PFAS concentrations in freshwater, seawater, and stormwater typically depend on proximity to the release point and source concentrations. In addition to releases associated with identified sources, stormwater runoff from nonpoint sources can cause significant surface water contamination with PFAS.
Sorption of PFAS to suspended solids may affect PFAS concentrations in surface water. Suspended microplastic can also influence PFAS in surface water.
What is known about PFAS in wastewater?
In addition to domestic wastewater, municipal wastewater treatment plants also collect and treat wastewater from indirectly discharging commercial enterprises. If PFAS are contained in commercial wastewater, they enter the treatment plants in this way. PFASs are only partially retained in state-of-the-art mechanical-biological wastewater treatment plants and are therefore detectable both in wastewater treatment plant effluents and in sewage sludge.
PFAS in Germany – a serious threat to the drinking water supply
In 2006, scientists from the Institute of Hygiene at the University of Bonn discovered high concentrations of perfluorooctanoic acid (PFOA) in the Ruhr River, which could be traced back to the application of a waste mixture containing PPAS as a supposed soil conditioner on arable land.
Following the discovery of this environmental scandal in North Rhine-Westphalia, it was quickly recognized in Germany that PFAS pose a serious threat to drinking water supplies. Since then, with the help of systematic investigations, numerous other PFAS groundwater contamination incidents have been discovered, in some cases related to the use of PFAS in the textile, paper, photographic and electroplating industries, but mostly caused by extinguishing agent foams or illegal dumping of PFAS-containing wastes.
The largest area of damage in Germany is located in Central Baden, in a total area of about 750 hectares, distributed over several areas, in the city of Baden-Baden and the district of Rastatt.
What processes are there for reducing and removing PFAS from (waste)water?
Conventional biological (for example, microbiological degradation or mass transfer) and chemical processes (for example, hydrolysis, photolysis, oxidation, and reduction) are not suitable for removing PFAS from the environment because of the strong carbon-fluorine bond and their mobility.
Likewise, classical sand filtration, currently the last purification step in water treatment plants in industrialized countries, is unsuitable for PPAS removal.
Modern filtration techniques such as nanofiltration and reverse osmosis can remove between 90 and 99% of PFAS from wastewater. However, these methods are very cost-intensive because they require a minimum concentration of the compounds to be filtered, which can only be achieved by pretreating the waters to be purified.
Electrochemical oxidation processes are particularly suitable for high PFAS concentrations above 5 mg/l and are thus only suitable for the removal of industrial waters containing PFAS. Due to the formation of undesirable by-products such as adsorbable, organically bound halogens as well as bromate and perchlorate ions during the oxidation process, an additional cleaning step must subsequently be carried out, which represents a further disadvantage.
State-of-the-art: purification processes for PFAS in groundwater and drinking water
There is a consensus in the literature that sorption methods are the most effective and economical options for PFAS reduction in waters. Adsorbents achieve high adsorption capacities, are at the same time inexpensive to produce and reusable due to their regeneration capability.
Classical sorbents such as activated carbon and commercially available ion exchangers have been intensively investigated for PFAS removal. The advantage of activated carbon lies in its cost efficiency, versatile application possibilities (use as powdered activated carbon (PAH) or granulated activated carbon (GAH)) and relatively high, albeit only short-term, adsorption capacities towards PFOA and PFOS. The regeneration of activated carbons is carried out by chemical and biological means, which is why reuse is economically unviable and safe landfilling is necessary.
Although the remediation of a 10-hectare PFAS-contaminated area in the Hochsauerlandkreis (Brilon-Scharfenstein) with activated carbon has already been reported, the use of GAH in drinking water treatment plants showed fluctuating, hardly reproducible and highly variable reduction rates of -220 to 5 % for PFOA and -185 to 98 % for PFOS. Already in 2007, Lampert et al. showed higher adsorption capacities of various anion exchange resins compared to GAH. Due to the high acquisition and manufacturing costs, the aim is to reuse the adsorbers by regeneration. This is done by adding methanol, which is, however, cost-intensive and ecologically disadvantageous.
According to the current state of the art, the processes activated carbon adsorption, ion exchange, flocculation and membrane processes are sometimes more and sometimes less suitable for the purification of groundwater containing PFAS. For the wastewater treatment plant or landfill leachate application, the data are incomplete. However, due to the complexity of the water composition and the influence of additional interfering ions and particles (in particular cations, DOC, water hardness), it is to be expected that treatment with conventional combinations will not be cost effective and / or ecologically justifiable.
It currently appears that only two processes, namely activated carbon adsorption and the membrane process, are capable of reducing even short-chain PFAS to concentrations of '10 ng/L in groundwater, but – and this is an important aspect – there are no comprehensive, reproducible data sets available that compare the target and non-target analytics of the PFAS compounds.
It should also be noted that both the ion exchange and precipitation processes currently require an additional activated carbon stage for post-purification in order to achieve low PFAS concentrations at all.
When using cost-intensive membrane processes, about 15-20% of the treated volume flow is produced as concentrated retentate, which has to be treated separately. Retentate containing PFAS can be purified with the aid of activated carbon, but this process step is also associated with high costs and is currently not yet profitable. The use of flocculants produces sludge containing PFAS, which must be disposed of as hazardous waste in a high-temperature incinerator. Resource efficiency and sustainability are not yet given in this process solution. In the case of ion exchangers, the regeneration of the systems is the limiting step. They can only be incompletely regenerated with NaCl or NaOH solutions. The resulting regenerate must be subjected to further purification, usually activated carbon adsorption. In the course of operation, the absorption capacity of the ion exchange resin decreases, so that it must eventually also be disposed of in a high-temperature incinerator.