Sports and Microplastics
27. June 2023
Sustainable Blue Economy
5. November 2023Forever Chemicals: What is behind perfluorinated and polyfluorinated alkyl substances - or PFAS for short? (Part 1/2)
From non-stick pans to waterproof clothing, PFAS (per- and polyfluoroalkyl substances) seem to have revolutionized modern living, but are a major concern due to their high persistence (resistance to degradation), toxicity and harmful environmental and health effects.
These “forever chemicals” have been used in industry since the 1940s and were already detected in human blood in the 1970s. PFAS contaminate the air, water, and soil along the entire value chain. Recently, it was even found that rainwater in most locations around the world contain PFAS levels that greatly exceed safety levels.
In February 2023, ECHA released its proposal for a PFAS ban. Exemptions are already being discussed, but the problem is known and solutions are rare, yet urgently needed. In our two-part blog, we dive deeper into the matter, with both assessments and outlooks.
PFAS has become a buzzword for yet another type of environmental pollution these days, but what are they?
PFAS (per- and polyfluoroalkyl substances) are a large class of over 9000 synthetic (man-made) non-aromatic, organic chemicals, although the number of PFAS chemicals continues to grow, as more are developed and produced. They are widely used across industries for their water-, stain-, and grease-repellent properties, their thermal and electrical insulation, and durability under extreme conditions.
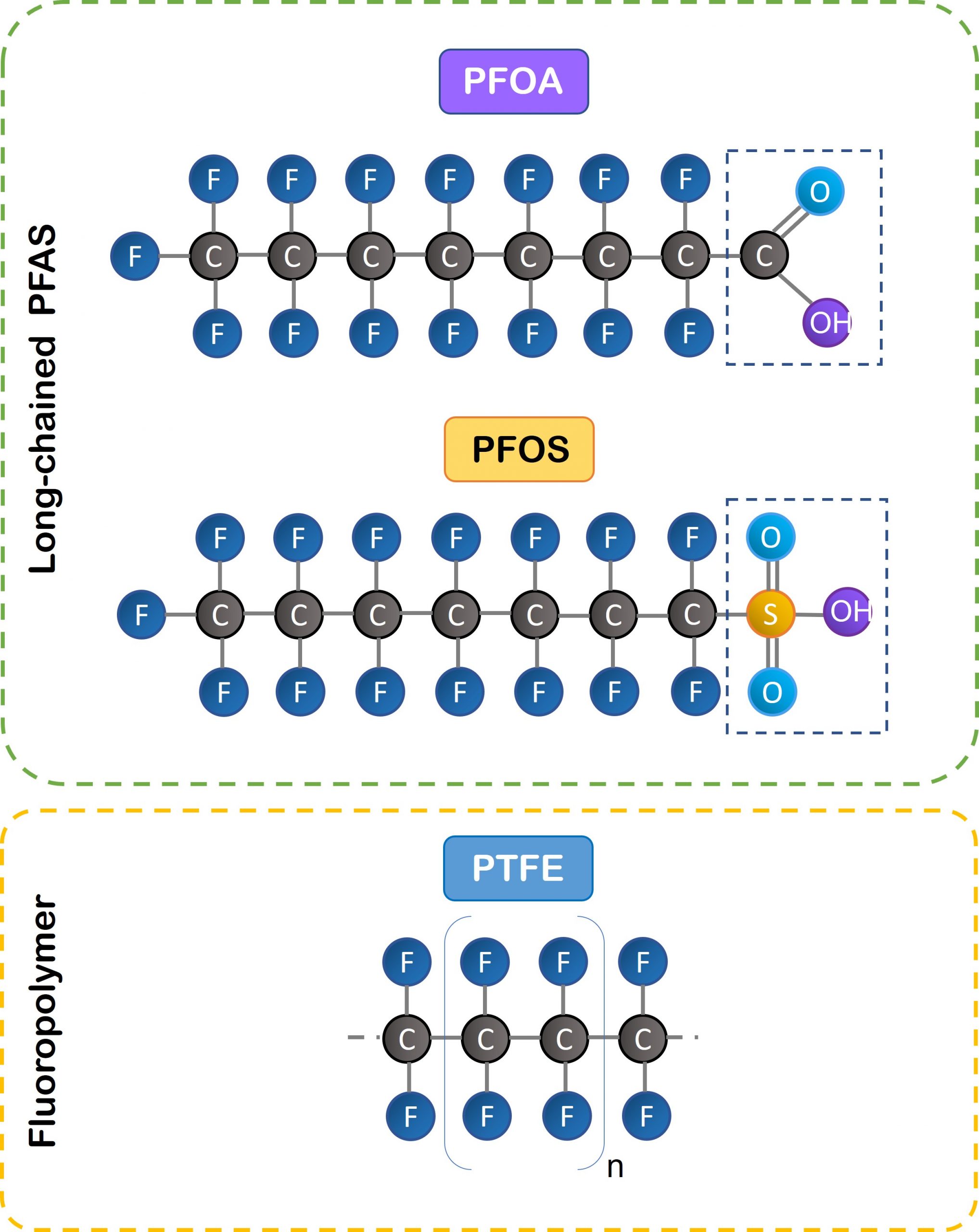
This is the PFAS definition given in ECHA’s proposed restrictions (consistent with the July 2021 Organization for Economic Cooperation and Development (July 2021 OECD): Substances that contain at least one fully fluorinated methyl (CF3-) or methylene (-CF2-) carbon atom, without any H / Cl / Br / I attached to it.
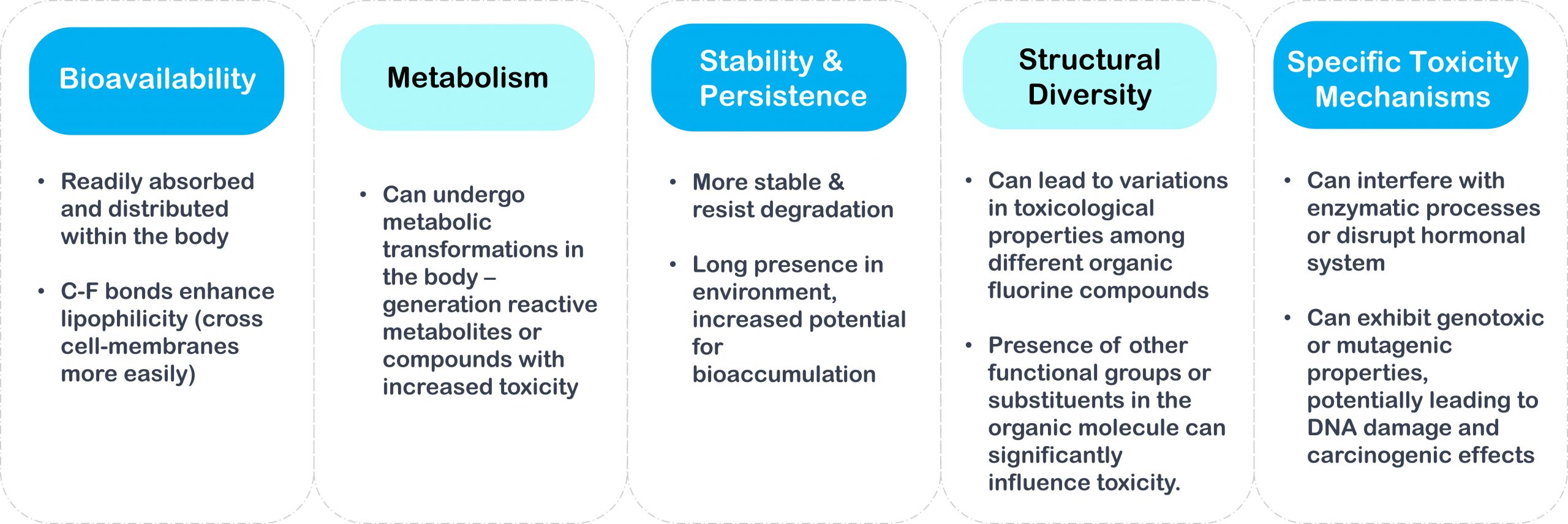
What is chemically behind PFAS and what is the reason for the higher toxicity of organic fluorine-containing compounds compared to inorganic compounds?
PFAS molecules have a chain of linked carbon and fluorine atoms, with at least one fully fluorinated carbon atom (all hydrogen atoms have been replaced by fluorine atoms). This is one of the strongest bonds in organic chemistry, which makes them highly resistant to thermal, chemical, or biotic degradation. But this durability means they are also nearly indestructible. So, what is released into the environment accumulates and persists.
PFAS are classified as short- or long-chain, according to the number of carbon atoms in the PFAS structure, whereby long-chain PFAS have six or more carbon atoms.
Two of the most widely used and studied PFAS until now are Perfluorooctanoic acid (PFOA) and Perfluorooctane Sulfonate (PFOS).
These long-chained, non-polymeric PFAS were commonly used in manufacturing and consumer goods (e.g. stain repellants, firefighting foams, industrial processes) due to their chemical characteristics and are well known to be problematic. Both have already been restricted under various European and international laws (PFOS since 2006 and PFOA since 2020).
Phasing out these compounds resulted in a decline in their levels in human blood, but the compounds continue to persist in the environment and in humans.
The problem with the PFAS compounds is multifaceted and the impact and long-term consequences are largely unknown
The same properties that make PFAS useful for many applications (i.e. grease-, water-, stain-, heat- and weather-resistant) also make them a huge environmental problem. They are extremely persistent against natural degradation processes- some PFAS take over 1000 years to degrade – and are often highly mobile, meaning they can be transported long distances or move readily into ground and surface waters. Further, due to their low degradation rates they accumulate in the environment, which in turn results in an increased exposure to humans and other species. This is a huge problem, as PFAS are toxic at extremely low levels (i.e. parts per quadrillion). And as PFAS are amphiphilic, they can bioaccumulate in the adipose tissues or bloodstream of organisms. with numerous negative effects on organisms and human health.
And remediating PFAS contamination is incredibly costly and hard to enforce. In Baden-Baden Germany, for example, the groundwater was poisoned through the application of fertilizer contaminated with PFC from 2006-2008 (the fertilizer was a mix of paper sludge and compost). This resulted in in one of the waterworks being shut down and the installation of filter systems, amounting to 6.5 million euros (as of 2022), with many more millions being needed in the future. Trials were still ongoing in 2022, with the polluter-pays-principle applying; however, remediating all 1200 hectares of land is not affordable or feasible.
What are the primary pathways of PFAS into the environment?
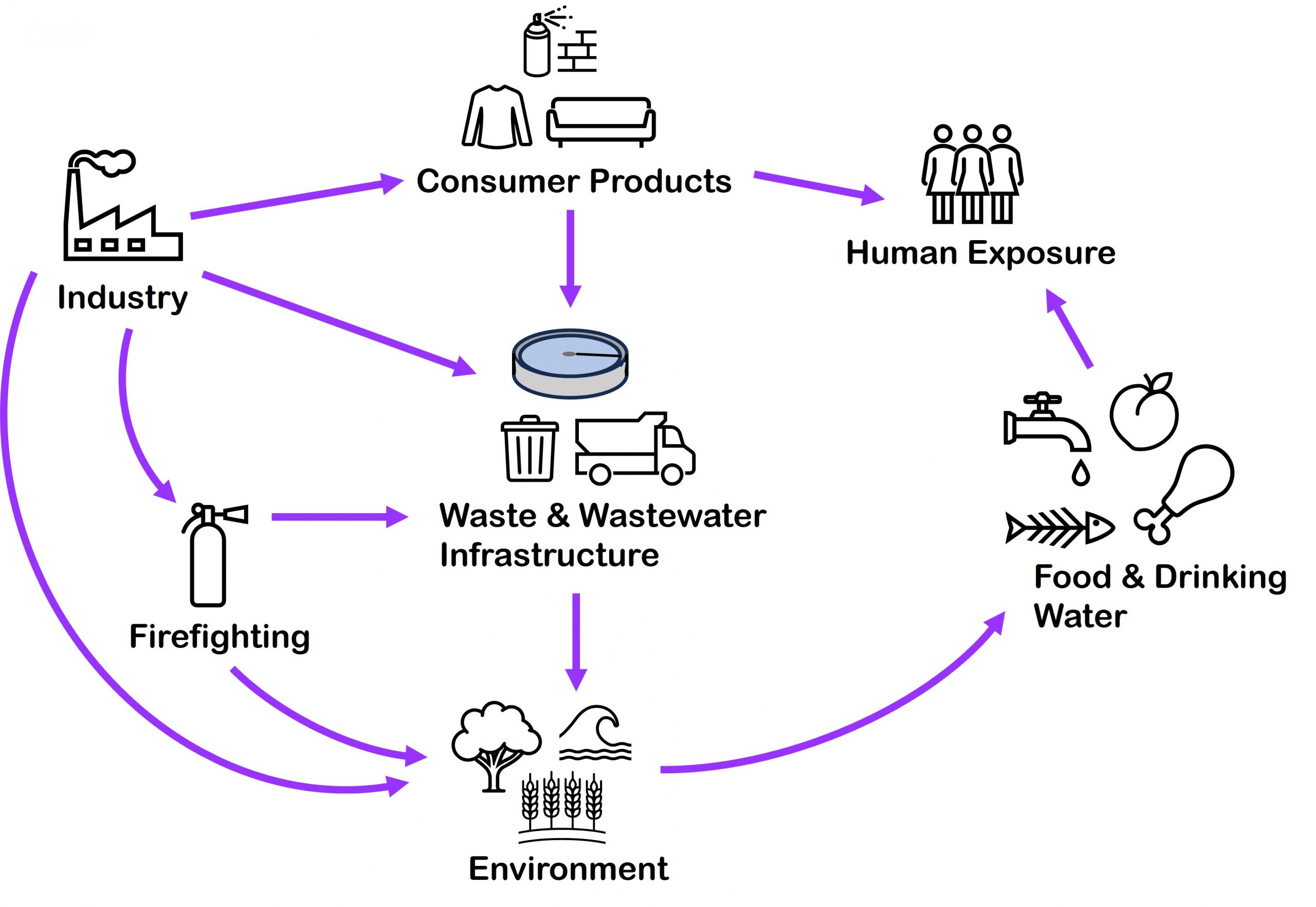
PFAS are released into the environment over the course of a product’s lifecycle – from production, through use, and disposal. It is estimated that 140,000 to 310,000 tonnes of PFAS were introduced to the EU market in 2020 alone.
Most PFAS in the urban environment come primarily from:
- Manufacturing and industrial sites that product PFAS containing products or use PFAS as part of their production process (g. textile waterproofing, metal finishing, carpet and furniture production, refrigerators and cleaning products)
- Fire training/fire response sites (with historical usage of aqueous film-forming foams (AFFF), particularly around civil and military bases, airfields)
- Industrial and municipal wastewater treatment plants -> originate from upstream sources such as industries, household products, human waste; available treatment options (including advanced treatment) do not destroy PFAS
- Solid waste management facilities (e.g., landfills) -> final repository of PFAS contaminated waste from manufacturing industries, sludge, and biosolids from WWTPs, foams from firefighting activities, and other materials such as coated textiles and cooking materials
Transport of PFAS mainly happens by air or when they are dissolved or dispersed in water. They can be released into the environment from the above sources via wastewater and stormwater discharges, surface water run-off, leaks and spills, and groundwater seepage, with water being the major environmental sink for both long- and short-chain PFAS.
Industrial and municipal wastewater as a source of PFAS
Conventional water and wastewater treatment plants are not able to effectively remove PFAS and are one of the main sources of PFAS into the environment. Studies have found that PFOA and PFOS levels were actually higher in the effluent than the influent for both industrial and municipal wastewaters. PFAS concentrations in industrial wastewater effluents range from 662-1143 ng/L, while the average concentrations of PFOA and PFOS in municipal wastewater effluents have been found to range from 10 to 100 ng/L, and 7-50 ng/L, respectively. But maximum concentrations up to 1 µg/L have been detected. These effluent concentrations, which typically flow directly into surface waters, are already exceeding the drinking water quality guidelines suggested by some countries, such as Australia, of 0.07 µg/L for PFOS and 0.56 µg/L of PFOA.
Because PFAS are resistant to biodegradation, they are not removed through conventional treatment processes, such as activated sludge. For groundwater and drinking water, advanced treatment processes such as activated carbon, ion-exchange processes, and membrane filtration are used for PFAS removal. However, the effectiveness of these technologies for wastewaters is impacted by the complex composition of wastewater and are not yet well studied.
How can we get a grip on the PFAS problem?
The answer: by acting quickly, economically, and ecologically and socially.
The first study results in the wastewater treatment plant environment have shown that some activated carbons and ion exchange resins can be effective in removing PFAS from wastewater. However, both technologies deliver high removal efficiencies only for some (specific) PFAS.
Their ability to remove short-chain PFAS is limited, sometimes not functional at all. In addition to the technical limitations, secondary pollutants and/or the high costs and problems associated with regeneration and disposal processes, especially for ion exchange resins and activated carbon, must also be included in any economic analysis.
It should also be clear to users that with both of these technologies, both chain length/functional group and the ions and organics present in the wastewater have a significant impact on the removal efficiency. All in all, the solutions show potential but lots of more work needs to be done, until varified data deliver the real impact (from sustainable process design, to ecologic and economic benefits).
What are we doing about PFAS?
The compound class of PFAS is not only highly interesting in terms of chemistry and environmental science, but also on our agenda in terms of technology development. In our research projects, we are working on new innovative approaches, which, among other things, are looking at the combination of Advanced Oxidation Process, the use of activated carbon and Wasser 3.0 PE-X®. Back in 2016, we already pursued new methods for the removal of PFAS in the university research project RE-Fluor-X together with abcr GmbH, but had to put the research work on hold due to a lack of funding.
The link between PFAS and microplastics is also on our agenda, as our agglomeration-fixation products / agglomerates can also act as carrier materials for other micropollutants, opening up a variety of new possibilities. And now perhaps you come into play, because:
We are looking for project partners with a specific PFAS/microplastic problem in e.g. industrial or municipal wastewater. Are there operators of analytical laboratories who are interested in implementing our analytical method for the comprehensive detection of PFAS compounds? You can find the relevant publications here.